enVigil-PnP: Monitoring System
enVigil-PnP provides a simple to use, cost effectivesolution which ensures a GMP/GLP compliant system and is ideally suited to small pharmaceutical production, hospital pharmacy and IVF clinic operations.
With a range of package sizes supporting up to 20 particle counters, 100 active air samplers and up to 160 environmental inputs enVigil-PnP is easy to deploy, verify and validate.
Place your order at AQUAANALYTIC, Latvia.
We will quickly deliver your order to any country.
Pharmagraph’s monitoring systems can start small, saving initial investment, then scale up as the facility grows, building on existing hardware and monitoring sensors and providing additional monitoring stations as the system grows. Initial systems can start as small as a single environmental parameter and scale up to an entire facility of parameters because of its modular nature.
Pharmagraph enVigil-PnP: Monitoring System
Demonstrates GMP/GLP compliance for cleanrooms and laboratories
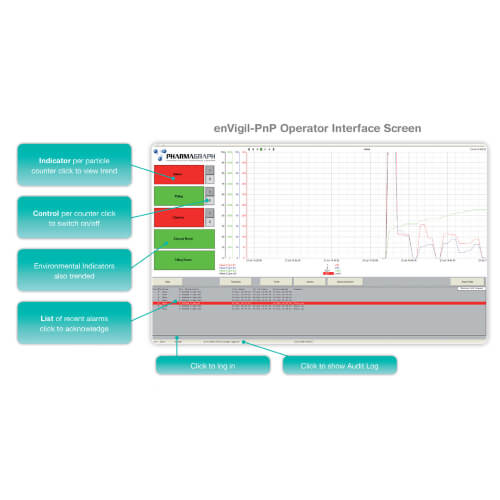
enVigil-PnP is provided with an overview menu with status indicators, trends, alarms, data logging, reports and data to MS Excel. Optional modules are also available to support instrument tracking, batch reporting, network view nodes, OPC server and GSM modem support for data on alarm to email and SMS text.
enVigil-PnP supports MetOne series remote and portable particle counters and can be additionally equipped with active air samplers and environmental sensors. Pharmagraph’s unique approach enables you to deploy a compliant monitoring system with minimal configuration and documentation by using standard building blocks. With the introduction of the highly capable enVigil PnP offering, you can now put together a system more rapidly than ever.
enVigil-PnP offers support for isolator gassing operations and particle counter “swap-ability” in order to minimise system downtime due to contamination failures. If you do not require particle counting, then enVigil-PnP offers a low-cost version for environmental or viable (active air sampling) monitoring only.
enVigil-PnP is complete with a simple Verification Protocol that includes a Functional Design Specification and Installation and Operational Acceptance Test Protocols allowing the enVigil-PnP system to be easily designed, configured, installed, commissioned and verified.
21 CFR Part 11
Compliance Assured
Pharmagraph software has been written from the ground up to comply with the tough demands of 21 CFR part 11 through the use of tight security, proprietary binary logging and provision of an audit trail. The software system is secured via unique usernames and passwords, with automatic log out when the system is left idle. A secure binary format is used for data log files making it virtually impossible to alter records. An audit trail logger automatically tracks changes to the system configuration, recording what has been changed, by whom, when and for what reason. The audit trail logger also records operator log in and log out activity, including failed log in attempts.
Request a quote
You can trust the 10-year experience of our engineers in striving to provide the best solutions for your business!

